
Discover the Exopulse Mollii Suit
Notification: The information contained on this site is only intended for an audience outside the United States. Please note that the products described herein are not FDA approved.
More possibilities for your patients with spasticity.
Discover a new breakthrough in wearable, near full-body neurostimulation: a drug-free, non-invasive technology designed to enhance patients’ mobility and relieve their spasticity-related pain.
Neurostimulation is a proven approach that has been extensively evaluated in neuromuscular disorders like spasticity (1-6) – a debilitating condition associated with cerebral palsy (CP), multiple sclerosis (MS) and stroke. The Exopulse Mollii Suit takes this trusted technique a groundbreaking step closer to its true potential for people with spasticity.
Targeted neurostimulation tailored to each patient’s unique needs.
Just like you and your clinical team, the Exopulse Mollii Suit provides personalized care for every patient. With 58 embedded electrodes, each controlled by up to 30 settings, you can precisely target stimulation to up to 40 key muscle groups throughout the body – and potentially deliver significant improvement in patients’ mobility and pain symptoms in as little as an hour. (6)
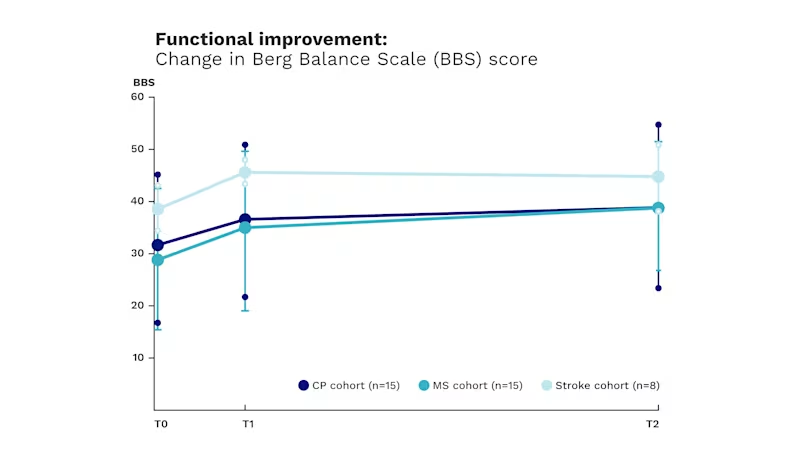
Give your patients a chance to move more freely and safely. (6)**
Early clinical results show that patients with CP, MS, and stroke had better balance and reduced their risk of falls after just 60 minutes in the Exopulse Mollii Suit. Regular, every-other-day stimulation helped them maintain their improvement.
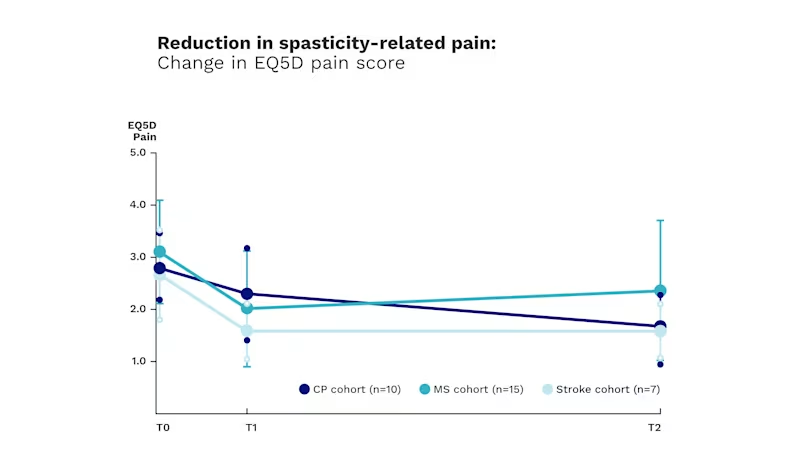
Help patients achieve swift, sustained relief from spasticity-related pain. (6)***
In the same preliminary study, an hour in the Exopulse Mollii Suit helped significantly reduce pain symptoms in patients who reported them. Four weeks later, patients who regularly used the Suit experienced continuing reduction in pain.
Exopulse Mollii Suit clinician brochure.
Take a snapshot of the technology, evidence, and start-up steps for your clinic.
The Exopulse Mollii Suit. (HQ)
1 rezultata od ukupno 1
More important information for healthcare professionals.
Select a topic below to learn more about this breakthrough approach to neurostimulation, and how it fits into both your clinical practice and your patients’ spasticity care.
Get started with the Exopulse Mollii Suit.
Get started with the Exopulse Mollii Suit.
*The testimonials, statements, and opinions presented in the videos are only applicable to the individuals depicted. The testimonials are not representative of patient experience. The exact results and experience will be unique and individual to each patient. The users depicted in the videos received compensation for the time spent on filming with Ottobock.
Not all products and services are registered or available for sale in all countries.
**Based on change in Berg Balance Scale (BBS) score in an open-label study of group-level response to Suit stimulation in patients with CP (adult and pediatric), MS, and stroke. Results of this study were evaluated in 38 patients with impaired balance and an increased fall risk (n=15/15/8 CP/MS/stroke, baseline BBS score < 45). Patients in all cohorts reported significant improvement in BBS score after 60 minutes of stimulation (T1) and after 4 weeks of stimulation every other day (T2).
***Based on change in EQ5D pain levels in an open-label study of group-level response to Suit stimulation in patients with CP (adult and pediatric), MS, and stroke. Change in pain levels was evaluated in a subset of 32 patients who reported spasticity-related pain at baseline (n=10/15/7 CP/MS/stroke, baseline EQ5D pain > 1). Patients in all cohorts reported significant improvement in EQ5D pain score after 60 minutes of stimulation (T1) and after 4 weeks of stimulation every other day (T2).
References: 1. Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of Spasticity After Spinal Cord Injury: Current Techniques and Future Directions. Neurorehabil Neural Repair. 2010; 24(1):23-33. DOI: 10.1177/1545968309343213. 2. Rabchevsky AG, Kitzman PH. Latest approaches for the treatment of spasticity and autonomic dysreflexia in chronic spinal cord injury. Neurotherapeutics. 2011; 8(2):274-282. DOI: 10.1007/s13311-011-0025-5. 3. Stein C, Fritsch CG, et al. Effects of Electrical Stimulation in Spastic Muscles After Stroke: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stroke. 2015; 46(8):2197-2205. DOI: https://doi.org/10.1161/STROKEAHA.115.009633. 4. Bosques G, Martin R, et al. Does therapeutic electrical stimulation improve function in children with disabilities? A comprehensive literature review. J Ped Rehab Med. 2016; 9(2):83–99. DOI: 10.3233/PRM-160375. 5. Etoom M, Khraiwesh Y, et al. Effectiveness of Physiotherapy Interventions on Spasticity in People With Multiple Sclerosis: A Systematic Review and Meta-Analysis. Am J Phys Med Rehab. 2018; 97(11):793-807. DOI: 10.1097/PHM.0000000000000970. 6. Exopulse Registry Clinical Research Report; Data on File.

