

Put on possible: The exopulse suit for spasticity & symptoms of fibromyalgia.
Explore the latest features & updates.
Notification: The information contained on this site is only intended for an audience outside the United States. Please note that the products described herein are not FDA-approved.
An innovative wearable approach to neuromodulation.
It’s a method you trust for many complex conditions, especially when your patients need a drug-free, non-invasive way to complement their primary treatments. Now, with the latest exopulse suit, it’s an even more powerful option for patients with spasticity and fibromyalgia.
At every step, the suit works alongside standard therapies for both conditions, delivering effective neuromodulation anywhere your patients need it. Discover how it can help you create new possibilities for their care.
Newly redesigned, it’s a more user-friendly suit.
This unique assistive device delivers near-full body stimulation that helps reduce spasticity symptoms typical of conditions like cerebral palsy (CP), multiple sclerosis (MS), and stroke—as well as symptoms of fibromyalgia.
The latest design sports a range of valuable new features and enhancements for both users and clinicians—but still delivers the same quick, sustained relief.

Even more precise stimulation
The redesigned suit now features an optimized array of 50 enlarged, combined electrodes that can be well positioned over the belly of target muscles.
The redesigned suit now features an optimized array of 50 enlarged, combined electrodes that can be well positioned over the belly of target muscles.
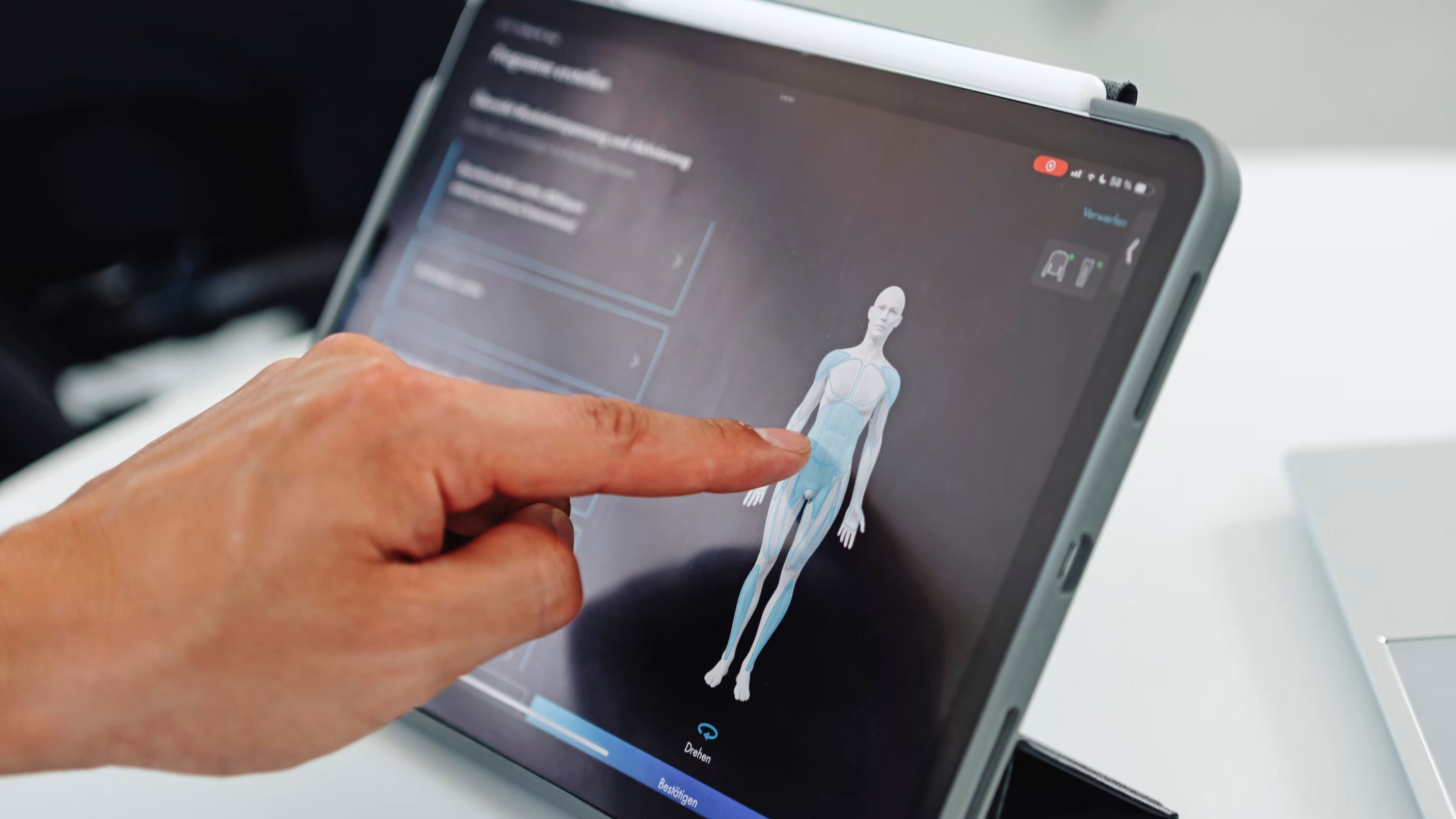
Simplified digital setup process
A new clinician app now lets you connect to the control units via Bluetooth and complete setup in just a few minutes.
A new clinician app now lets you connect to the control units via Bluetooth and complete setup in just a few minutes.

Even easier donning & doffing
The jacket and pants are now made from a stretchier single-layer fabric that makes the garments even more comfortable to put on, take off, and wear.
The jacket and pants are now made from a stretchier single-layer fabric that makes the garments even more comfortable to put on, take off, and wear.

Easier configuration & personalization
The garments now have separate, rechargeable control units that eliminate the hassle and waste of disposable batteries. Both units can be accessed and adjusted with either app.
The garments now have separate, rechargeable control units that eliminate the hassle and waste of disposable batteries. Both units can be accessed and adjusted with either app.

Adjustable stimulation for users
Once you’ve set up the device, patients can use their app to make small adjustments to their stimulation when needed—freeing up valuable clinic time for you and your team.
Once you’ve set up the device, patients can use their app to make small adjustments to their stimulation when needed—freeing up valuable clinic time for you and your team.

Even more eligible users at your clinic
The suit’s new control unit configuration eliminates magnetic connections that can interfere with implanted devices.
The suit’s new control unit configuration eliminates magnetic connections that can interfere with implanted devices.
Put life back in motion for patients with spasticity.*
Neuromodulation is a proven approach that has been extensively evaluated in neuromuscular conditions like spasticity [1-6]—a debilitating condition associated with CP, MS, and stroke.
The exopulse suit takes this trusted technique a step closer to its true potential. It uses reciprocal inhibition to release spastic muscles, rebalance muscle activity, and help patients move more freely and with less pain.
Move more freely.*
Clinical results show that patients with CP, MS, and stroke had better balance and reduced their risk of falls after just 60 minutes in the exopulse suit. Regular, every-other-day stimulation helped them maintain their improvement. [6]
![[B2B] EXOPULSE 9.5: (SP + FM) - Help patients move more freely](https://images.ctfassets.net/8ks1shyq5m87/5qLgblF7oSUqZhOrEt8bza/724fcd0a1f1447764e61cb2a3c0eebed/4881751_BBS_blue_169.jpg?fm=avif&fit=fill&f=faces&w=800&h=450&q=75)
Deliver swift, sustained relief from spasticity-related pain.***
In the same study, an hour in the exopulse suit helped significantly reduce pain symptoms in patients who reported them. Four weeks later, patients who regularly used the suit experienced continuing reduction in pain. [6]
![[B2B] EXOPULSE 9.5: (SP + FM) - Deliver swift, sustained relief](https://images.ctfassets.net/8ks1shyq5m87/43J8l4DbYlDCuDWnXbLKbz/8aaf29230ec5aeffd5db4562c2ffe9a7/4881753_pain_blue_169.jpg?fm=avif&fit=fill&f=faces&w=800&h=450&q=75)
Proven fibromyalgia relief that increases with time.
With this complex condition, clinical success takes care that’s tailored to each patient’s unique needs. Studies agree: That’s exactly what the suit offers for patients with fibromyalgia. [7,8]
It helps reduce pain by activating nervous system mechanisms that block debilitating symptoms before they reach the central nervous system. In a randomized, double-blind, sham-controlled clinical trial, the suit significantly reduced users’ fibromyalgia symptoms in just two weeks—with further improvement after four. [7]
Open-label study (n=50)
Rapid pain relief in 60 minutes
In a study of patients’ response to a single hour of stimulation, some users reported feeling
significantly less fibromyalgia-related pain after just one session in the suit.
Randomized, sham-controlled study (n=33)
Significant relief in just two weeks³
With exopulse suit after 2 weeks of daily usage compared to baseline, active condition
(phase 1; no sig. changes for sham condition reported):
Pain: 14% reduction in VAS pain scale, 17% FIQ pain subscale, 16% in BPI pain interference subscale
Fibromyalgia impact: 18% reduction in total FIQ score, 19% in FIQ physical impairment and 17% in FIQ
fatigue subscalesQuality of life: 47% improvement in SF-36 bodily pain and vitality subscales
Depression: 25% decrease in FIQ anxiety score and 13% in HADS anxiety score 64% improvement in Global Clinical Impression
![[B2B] EXOPULSE 9.5: (SP + FM) - Fibromyalgia - Image 1 (HQ)](https://images.ctfassets.net/8ks1shyq5m87/1uHb33HIWAsaBwkrhezjyh/4ef76a04bdd7ecd80ac47aa5237ec04d/Treatments_benefits_over_time_169_I.jpg?fm=avif&fit=fill&f=center&w=800&h=450&q=75)
Increased relief with regular use³
With exopulse suit after 4 weeks of daily usage compared to baseline, open label phase (phase 2):
Pain: 25% reduction in VAS & FIQ pain scales, 16% in BPI pain severity and 17% in BPI pain
interference subscalesFibromyalgia impact: reduction in 21% total FIQ score, 20% FIQ fatigue subscale
Quality of life (SF-36): 20% increase in social functioning, 35% in health change, 54% in vitality, 92% in
role emotional and 161% in role physical subscalesDepression: 26% reduction in FIQ anxiety, 14% reduction in HADS anxiety and 12% in HADS
depression subscales79% of users demonstrated improvement in global clinical impression
Physical and psychological relief use³
After 4 weeks of daily stimulation, users also reported significant improvement in fibromyalgia related
symptoms of anxiety and depression³
![[B2B] EXOPULSE 9.5: (SP + FM) - Fibromyalgia 2 - Image (HQ)](https://images.ctfassets.net/8ks1shyq5m87/4oTfE2ajvneekv3fRu1DmL/d47c9fcbac053590d83179381ed2c504/Treatments_benefits_over_time_169_II.jpg?fm=avif&fit=fill&f=faces&w=800&h=450&q=75)
Relieve physical and psychological symptoms.
For fibromyalgia patients, chronic pain can have a big impact on mental health too. With consistent use, the suit can help address many psychological symptoms too.
In fact, after 4 weeks of daily stimulation, patients report both increasing alleviation of pain and fatigue and improvement in depression and anxiety. [7]

Complement their current fibromyalgia treatment.
The suit can be safely used alongside any other standard therapy, including pharmaceuticals, physical therapy, and psychiatric care.
And unlike some neuromodulation devices, the suit requires no uncomfortable, invasive implantation procedures. Users simply slip on the garments and hit “start.”

See how the suit changed these users’ lives.
With a simple, non-invasive, drug-free way to manage their spasticity, Ricky, Lena and Jacqueline are rediscovering life’s possibilities.
More important information for healthcare professionals.
Evaluate the suit in your clinic.
Ready for a hands-on evaluation of the suit’s design, technology, and functionality? Contact our team of neuromodulation experts to schedule a virtual demo or in-person training.
All fields required.
References:
1. Elbasiouny SM, Moroz D, Bakr MM, Mushahwar VK. Management of Spasticity After Spinal Cord Injury: Current Techniques and Future Directions. Neurorehabil Neural Repair. 2010; 24(1):23-33.
2. Rabchevsky AG, Kitzman PH. Latest approaches for the treatment of spasticity and autonomic dysreflexia in chronic spinal cord injury. Neurotherapeutics. 2011; 8(2):274-282.
3. Stein C, Fritsch CG, et al. Effects of Electrical Stimulation in Spastic Muscles After Stroke: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Stroke. 2015; 46(8):2197-2205.
4. Bosques G, Martin R, et al. Does therapeutic electrical stimulation improve function in children with disabilities? A comprehensive literature review. J Ped Rehab Med. 2016; 9(2):83–99.
5. Etoom M, Khraiwesh Y, et al. Effectiveness of Physiotherapy Interventions on Spasticity in People With Multiple Sclerosis: A Systematic Review and Meta-Analysis. Am J Phys Med Rehab. 2018; 97(11):793-807.
6. Hahn A, Moeller S, et al. Effects of a full-body electrostimulation garment application in a cohort of subjects with cerebral palsy, multiple sclerosis, and stroke on upper motor neuron syndrome symptoms. Biomedical Engineering / Biomedizinische Technik. 2024; 69(1):49-59.
7. Mattar JG, Chalah MA, Ouerchefani N, Sorel M, Le Guilloux J, Lefaucheur JP, Abi Lahoud GN, Ayache SS. The effect of the EXOPULSE Mollii Suit on pain and fibromyalgia-related symptoms-A randomized sham-controlled crossover trial. Eur J Pain. 2024 Sep 18. doi: 10.1002/ejp.4729. Epub ahead of print. PMID: 39291602.
8. Riachi N, Chalah MA, Ahdab R, Arshad F, Ayache SS. Effects of the TENS device, Exopulse Mollii Suit, on pain related to fibromyalgia: An open-label study. Neurophysiol Clin. 2023 Aug;53(4):102863.
*The testimonials, statements, and opinions presented in the videos are only applicable to the individuals depicted. The testimonials are not representative of patient experience. The exact results and experience will be unique and individual to each patient. The users depicted in the videos received compensation for the time spent on filming with Ottobock.
**Based on change in Berg Balance Scale (BBS) score in an open-label study of group-level response to suit stimulation in patients with CP (adult and pediatric), MS, and stroke. Results of this study were evaluated in 38 patients with impaired balance and an increased fall risk (n=15/15/8 CP/MS/stroke, baseline BBS score < 45). Patients in all cohorts reported significant improvement in BBS score after 60 minutes of stimulation (T1) and after 4 weeks of stimulation every other day (T2).
***Based on change in EQ5D pain levels in an open-label study of group-level response to suit stimulation in patients with CP (adult and pediatric), MS, and stroke. Change in pain levels was evaluated in a subset of 32 patients who reported spasticity-related pain at baseline (n=10/15/7 CP/MS/stroke, baseline EQ5D pain > 1). Patients in all cohorts reported significant improvement in EQ5D pain score after 60 minutes of stimulation (T1) and after 4 weeks of stimulation every other day (T2).